Yu Zhou,†,‡,# Jing Ma,§,# Xingyu Lin,† Xi-Ping Huang,∥ Kaichun Wu,*,§ and Niu Huang*,†
†National Institute of Biological Sciences, Beijing, No. 7 Science Park Road, Zhongguancun Life Science Park, Beijing 102206, China
‡Department of Pharmacology and Pharmaceutical Sciences, School of Medicine, Tsinghua University, Beijing 100084, China
§State Key Laboratory of Cancer Biology and Xijing Hospital of Digestive Diseases, Fourth Military Medical University, 127 West
Changle Road, Xi’an, Shaanxi Province 710032, China
∥Department of Pharmacology, The National Institute of Mental Health Psychoactive Drug Screening Program (NIMH PDSP), The
University of North Carolina, Chapel Hill, North Carolina 27759, United States
Abstract
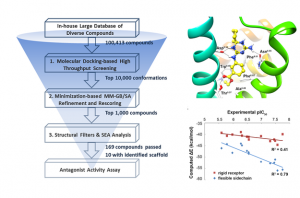
Here, we employed structure-based ligand discovery techniques to explore the opportunity of a recently determined crystal structure of the 5-Hydroxytryptamine 2B (5-HT2B) receptor. Ten compounds containing a novel chemical scaffold were identified; among them, seven molecules were active in cellular function assays with the most potent one exhibiting an IC50 value of 27.3 nM. We then systematically probed the binding characteristics of this scaffold by designing, synthesizing, and testing a series of structural modifications. The structure-activity relationship studies strongly support our predicted binding model. The binding profiling across a panel of 11 5-HT receptors indicated these compounds are highly selective for the 5-HT2B receptor. Oral administration of compound 15 (30 mg/kg) produced significant attenuation of visceral hypersensitivity in a rat model of irritable bowel syndrome (IBS). We expect this novel scaffold will serve as the foundation for the development of 5-HT2B antagonists for the treatment of IBS.