CryoEM structure of yeast cytoplasmic exosome complex.
Jun-Jie Liu1,2,*, Chu-Ya Niu1,*, Yao Wu3,*, Dan Tan3, Yang Wang1, Ming-Da Ye1, Yang Liu2,4, Wenwei Zhao4, Ke Zhou5, Quan-Sheng Liu5, Junbiao Dai6, Xuerui Yang4, Meng-Qiu Dong3, Niu Huang3 and Hong-Wei Wang1
- 1Ministry of Education Key Laboratory of Protein Sciences, Tsinghua-Peking Joint Center for Life Sciences, Beijing Advanced Innovation Center for Structural Biology, School of Life Sciences, Tsinghua University, Beijing 100084, China
- 2Joint Graduate Program of Peking-Tsinghua-National Institute of Biological Science, Tsinghua University, Beijing 100084, China
- 3National Institute of Biological Sciences, Beijing 102206, China
- 4MOE Key Laboratory of Bioinformatics, Tsinghua-Peking Joint Center for Life Sciences, Center for Synthetic and Systems Biology, School of Life Sciences, Tsinghua University, Beijing 100084, China
- 5The Multidiscipline Research Center, Institute of High Energy Physics of the Chinese Academy of Sciences, Beijing 100049, China
- 6MOE Key Laboratory of Bioinformatics, Center for Synthetic and Systems Biology, School of Life Sciences, Tsinghua University, Beijing 100084, China
Correspondence: Hong-Wei Wang, E-mail: hongweiwang@tsinghua.edu.cn
*These three authors contributed equally to this work.
Received 3 November 2015; Revised 26 January 2016; Accepted 7 March 2016
Advance online publication 13 May 2016
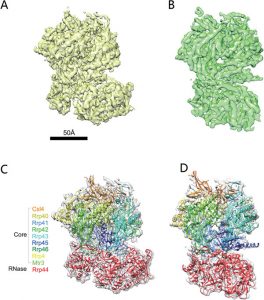
The eukaryotic multi-subunit RNA exosome complex plays crucial roles in 3′-to-5′ RNA processing and decay. Rrp6 and Ski7 are the major cofactors for the nuclear and cytoplasmic exosomes, respectively. In the cytoplasm, Ski7 helps the exosome to target mRNAs for degradation and turnover via a through-core pathway. However, the interaction between Ski7 and the exosome complex has remained unclear. The transaction of RNA substrates within the exosome is also elusive. In this work, we used single-particle cryo-electron microscopy to solve the structures of the Ski7-exosome complex in RNA-free and RNA-bound forms at resolutions of 4.2 Å and 5.8 Å, respectively. These structures reveal that the N-terminal domain of Ski7 adopts a structural arrangement and interacts with the exosome in a similar fashion to the C-terminal domain of nuclear Rrp6. Further structural analysis of exosomes with RNA substrates harboring 3′ overhangs of different length suggests a switch mechanism of RNA-induced exosome activation in the through-core pathway of RNA processing.